Unpacking the Promise of Gene-Based Therapies: Insights from EE’s Gene Therapy Town Hall
On August 22, 2023, Emily’s Entourage (EE) hosted a virtual town hall meeting that delved into the exciting world of gene-based therapies for cystic fibrosis (CF). This virtual event aimed to empower the CF community with knowledge about gene-based therapy trials and provide a venue for attendees to ask questions and receive answers from expert CF clinicians from some of the biggest CF clinics in the United States.
Our featured speakers included:
- Dr. Gregory Sawicki from Boston Children’s Hospital
- Dr. George M. Solomon from The University of Alabama at Birmingham
- Dr. Jennifer L. Taylor-Cousar from National Jewish Health
- Dr. Ahmet Z. Uluer from Boston Children’s Hospital/Brigham and Women’s Hospital
We were also honored to have Laura Mentch, a former gene-based therapy clinical trial study participant, offer a unique patient perspective. The town hall was moderated by EE’s Co-Founder, Emily Kramer-Golinkoff.
One thing was clear from the start – the CF community has burning questions about gene-based therapies. Questions were collected in advance (more than 400 questions/responses were submitted!) and also taken live during the town hall. More than 100 people from 5 different countries attended the live event, representing the concerns and hopes of our diverse CF community. You can view the town hall, which also included an overview presentation on gene-based therapies, in its entirety below.
Excitement Ahead
Here are some key takeaways and answers to the content and questions covered during the EE’s Gene Therapy Town Hall:
- Potential to reach all people with CF: Gene-based therapies (gene replacement, mRNA, gene editing) aim to “replace the problem” with replacement mRNA or genes or editing to correct mutations, making them potentially effective for all people with CF, regardless of underlying genetic mutation. However, for people with CF who have had organ transplants (lung, liver, kidney), the altered immune system resulting from transplant medications will exclude transplanted organ recipients from initial studies.
- Safety First: The presenters addressed the potential side effects of gene-based therapies. Multiple layers of safety are in place, and these therapies build on the foundations of previous CF treatments and learnings from other gene-based therapy studies. The main side effects are related to the inhaled substances (lipid nanoparticles are viruses) that are used to carry the new/corrected material into the lung cells. These substances may cause inflammation in the lungs, which can result in lung symptoms such coughing and chest pain, or full body systems such as fever. Understanding safety risks is crucial during the informed consent process.
- Impact Assessment: The impact of gene-based therapies compared to modulators is not yet known and likely depends on the specific therapy. If gene-based therapies could restore cystic fibrosis transmembrane conductance regulator (CFTR) function in all of the cells of the lungs, the scientific community would hope to see improvements in lung function and quality of life. However, a big question remains regarding what percentage of cells have to be corrected in order to reach a specific level of lung function response. It’s too early to know the answer to this question, but one possible goal of this current early stage of gene-based therapies is to help stabilize people in the final 10% until other, possibly better, therapies exist.
- Choosing Which Trial to Participate In: To choose between multiple trials, a person with CF should consult their physician, care team, and the primary investigator for each study. When deciding whether to participate, each person should consider scheduling demands, time commitments, and travel requirements. People outside the United States may have the opportunity to participate in gene-based therapy trials, although some barriers related to health insurance and logistics will apply.
- Realistic Timeline: Gene-based therapies are currently in early phase clinical trials, so it is difficult to predict how many years away they are from being fully approved treatment options. The pace of progress depends on trial enrollment and the early signs of safety and improvement that are shown in the trials. Trial progression has been slower than that of other recent examples of oral therapies in CF because these are brand new, never-before-tested therapies, so the FDA’s requirements have been rigorous and deliberate. For trials that target those in the final 10%, the community is small, which makes recruitment more challenging. The CF and scientific communities have to take these trials one day at a time; filling the trials is the first and most critical step.
Our panelists wrapped up the town hall by sharing what excites them most about gene-based therapies. Their enthusiasm was palpable. From excitement about the number of companies investing and advancing this research, to the tremendous amount of collaboration happening to drive these therapies forward – it is clear that there is lots of positive momentum in the field, which is absolutely critical to turning this research into a reality.
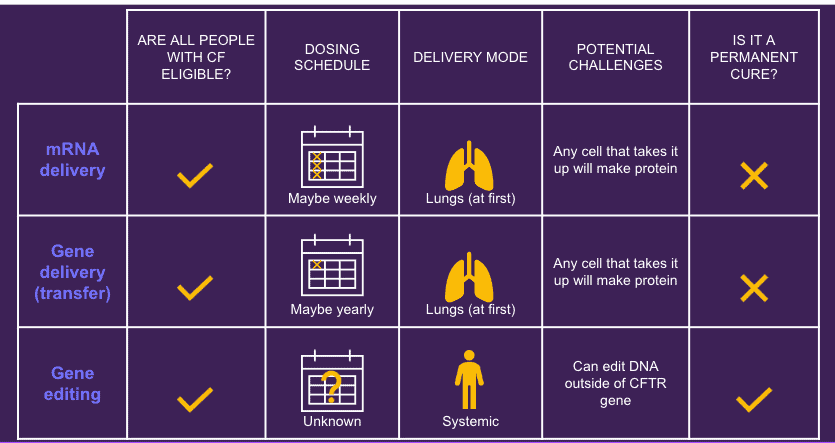
Original Image by Cystic Fibrosis Foundation, modified by Dr. George M. Solomon
Speeding Progress
It was clear in the town hall that one of the major hurdles to the development of new gene-based therapies is the time it takes to enroll participants. EE is working hard to overcome this hurdle — and you can help!
- If you or your child has at least one copy of a nonsense mutation of CF, consider signing up for EE’s patient registry. EE’s clinical trial matchmaking program utilizes our patient registry to inform people with CF about certain clinical trials that they may be eligible to participate in, equipping the CF community to make informed decisions and helping to speed the clinical trial recruitment process. With various gene-based therapy trials now enrolling participants – and more coming soon, this is an opportune time to sign up!
- You can also search for CF trials on the Cystic Fibrosis Foundation’s Clinical Trial Finder website or on ClinicalTrials.gov using keywords like “cystic fibrosis” and “gene therapy” or “mRNA.” More information may be available on dedicated clinical trial websites or through the respective companies.
- Stay informed, engage with your care team, and keep an eye on our EE newsletter and social channels for updates on our work supporting gene-based therapies.
For now, we’re excited about the potential these therapies hold, grateful for the vibrant CF community that’s driving progress forward, and eager to see these therapies come to fruition ASAP.
