Cystic Fibrosis Clinical Trial Matchmaking Program
One of the major barriers to therapeutic development is clinical trial recruitment, which can be costly, time-consuming, and inefficient. This is particularly true for people with rare diseases and mutations, like many of those in the final 10% of the cystic fibrosis (CF) community, because the pool of clinical trial participants is so small and spread out.
To expedite clinical trial recruitment, Emily’s Entourage (EE) created the CF Clinical Trial Connect: a global patient database exclusively for people with CF who are unable to benefit from CFTR modulators, including those with ineligible genetic mutations, side effects, or suboptimal response to CFTR modulators. The CTC database allows these individuals to contribute their data and become informed of relevant clinical research and trials. Utilizing data from the CTC, the Clinical Trial Matchmaking Program then connects individuals with CF to relevant clinical trial opportunities to speed clinical trial recruitment through targeted outreach from a trusted source.
What makes EE’s Clinical Trial Matchmaking Program unique?
Our CF Clinical Trial Matchmaking Program is direct-to-consumer, meaning that we share relevant clinical trial opportunities directly with individuals registered with the CTC database rather than going through clinics, care centers, or providers. EE’s support makes clinical trial recruitment faster, more targeted, and more efficient, enabling more expedited drug development. In addition, our Clinical Trial Matchmaking Program equips individuals with CF with the information and tools to pursue clinical research opportunities that may be right for them.
Getting into a clinical trial shouldn’t require that a person with cystic fibrosis be in the right place at the right time.
“Everyone with CF should have the opportunity to hear about relevant clinical trial opportunities and determine if it is the right opportunity for them to pursue. Through our Clinical Trial Matchmaking Program, we’re bridging the gap between trial opportunities and the people whose lives depend on them.” – Emily Kramer-Golinkoff, co-founder, Emily’s Entourage
We’ve seen firsthand that offering a ready-made community of willing trial participants helps attract biopharmaceutical companies to the CF space and inform trial site identification. The matchmaking program also promotes more strategic and equitable distribution of trials and representation among participants.
It is important to note that the privacy of our CF Clinical Trial Connect (CTC) participants is of critical importance to us. We do not share any personal data provided through the CTC database with companies or researchers, and will only ever share CTC data in aggregate.
Are you genetically ineligible for CFTR modulators or unable to benefit from existing modulator therapies? Sign up for CF Clinical Trial Connect (CTC).
Companies or investigators interested in our Clinical Trial Matchmaking Program can email registry@emilysentourage.org for additional information.
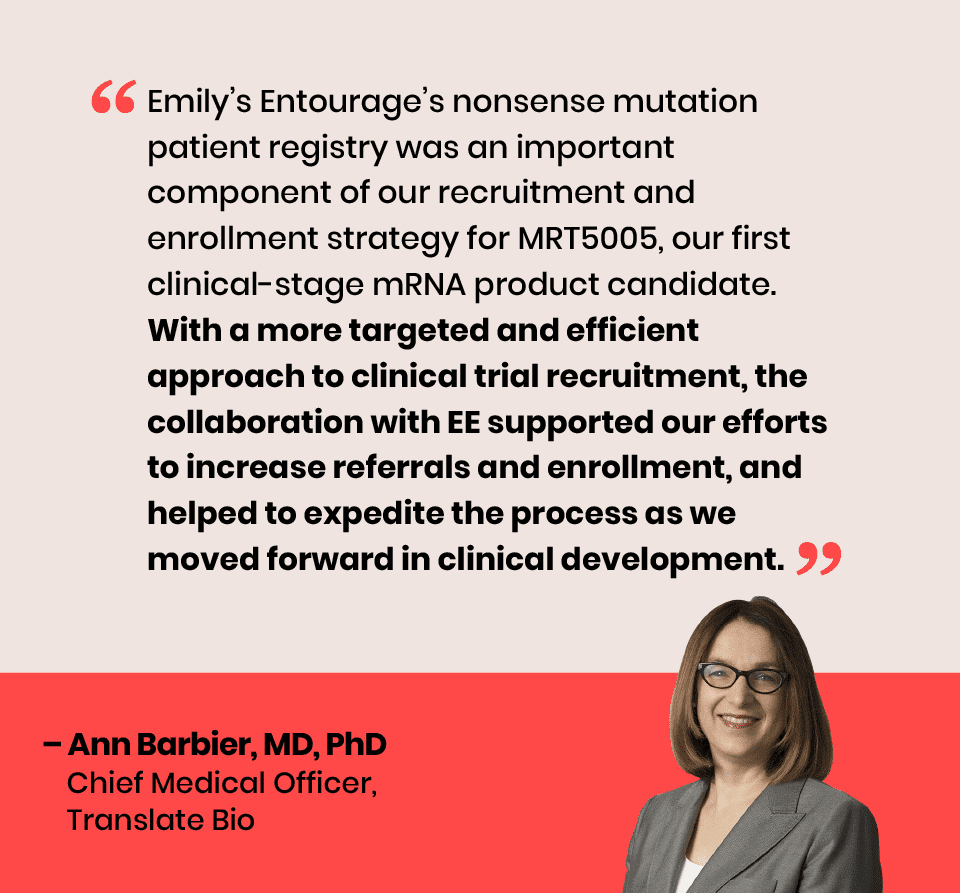
EE launched a first-of-its-kind collaboration with Translate Bio for the company’s MRT5005 mRNA trial. The partnership yielded increased screening and successful trial enrollments toward the company’s estimated enrollment goal of 40 participants.
