00

- Maison
- L'histoire d'Emily
- À propos de nous
- Recherche
- Événements
- Presse et médias
- Agir
- Blog
- Contact
- Faire un don

Subventions et investissements stratégiques
A Seismic Shift, Leadership for the Future
For many years, EE has been working hard to advance clinical trials for the final 10% of the cystic fibrosis (CF) community. Clinical trials represent the final major step in bringing novel therapies to people with CF and—until very recently—there were none for individuals in the CF population who do not benefit from currently available CFTR modulators.
That all changed throughout the past two years. Research projects that EE funded and helped support advanced in the pipeline and entered the clinic. Trials are no longer just “coming”; they are here. As of 2023, there were multiple trials beginning or about to begin for those in the final 10% of the CF community; EE was involved in virtually all of them.
This is a monumental development for multiple reasons: it demonstrates EE’s ability to identify and advance promising research, and it shows that therapies for the final 10% are moving forward and getting closer to people with CF. Actual studies—funded and supported by EE—are being conducted on individuals in the final 10% of the CF community. This is a seismic, critical shift in the CF space and marks a major milestone in our collective impact as an organization.

Chandra Ghose, PhD, Chief Scientific Officer at EE
With so much accomplished and so much more in the research pipeline, EE recognized the need for an experienced, visionary leader to guide our aggressive research and drug development efforts. The appointment of Chandra Ghose, PhD—an accomplished and award-winning scientist, entrepreneur, and founder, with sweeping experience in biomedical research—in January 2022 as Emily’s Entourage first-ever Chief Scientific Officer (CSO) marked a major step forward not only in advancing EE’s research goals, but in taking our organization to a completely new level of scope and impact.
Under Ghose’s leadership, EE has expanded its scientific prowess. Since joining EE, Ghose quickly went to work meeting with EE’s Scientific Advisory Board (SAB), biopharmaceutical companies, and the CF and EE communities to build and cultivate alliances with key stakeholders. Additionally, she spearheaded the development of a comprehensive, two-part “Scientific Roadmap” for EE. This strategic plan, highlighted in greater detail below, is designed to accelerate research and development and identify key pathways to achieve EE’s goals of fast breakthroughs and, ultimately, a cure for individuals in the final 10% of the CF community.
The result is an unprecedented level of direction and momentum in EE’s research and development activities and the harnessing of a transformative moment for both EE and the CF community. Together, the launch of various clinical trials, Ghose’s appointment as EE’s CSO, and the introduction of our new scientific roadmap reflect an affirmation of our past achievements and the promise of a future filled with more frequent, focused, and expedited advances for the final 10%.
Roadmap Overview
One of the significant early achievements of Ghose’s tenure has been the development of a two-part “Scientific Roadmap” to steer EE’s research and development work. Crafted with input from more than 35 researchers, industry leaders, clinicians, and people affected by CF, and supported by a thorough review and analysis of the scientific literature and clinical trials landscape, Part 1 of the Roadmap is designed to guide the creation of a robust, sustained pipeline of novel therapeutics for individuals in the final 10%. These research priority areas include:
- Mutation-specific small molecule rescue treatments for premature termination codons.
- Genetic therapies for premature termination codons.
- Non-viral vectors for DNA and RNA delivery to lung cells.
- Treatment and prevention of Staphylococcus aureus and other clinically important pathogens in the CF patient.
- Drug repurposing using a systems biology approach.
- Promising early stage research.
 In addition to determining priority research and therapeutic development areas, the roadmap also elaborates on EE’s strategies in venture philanthropy, clinical development, strategic and regulatory partnerships, and conferences. It continues to strategically guide EE’s core research and drug development efforts, while also enhancing all the ancillary supporting activities necessary to bring a therapy to market. It positions EE as a pivotal driving force and resource for the final 10% of the CF community, enhancing its ability to connect researchers, scientists, clinicians, industry leaders, venture philanthropists, and people with CF as well as their families.
In addition to determining priority research and therapeutic development areas, the roadmap also elaborates on EE’s strategies in venture philanthropy, clinical development, strategic and regulatory partnerships, and conferences. It continues to strategically guide EE’s core research and drug development efforts, while also enhancing all the ancillary supporting activities necessary to bring a therapy to market. It positions EE as a pivotal driving force and resource for the final 10% of the CF community, enhancing its ability to connect researchers, scientists, clinicians, industry leaders, venture philanthropists, and people with CF as well as their families.
Bringing lifesaving breakthroughs to individuals in the final 10% of the CF community requires more than just funding promising research. As such, Part 2 of the Roadmap focuses on the multiple key, concurrent pathways essential for achieving EE’s research and drug development goals. The pathways include: Academic Research, Venture Philanthropy, Clinical Development, Regulatory, Strategic Partnerships, and Conferences. EE is focused on clearing a path to ensure that drug development continues full-speed ahead and assets are able to move through the various stages seamlessly and expeditiously.
Phage at the Forefront
Among the most notable recent clinical advances resulting from EE’s grant funding involve phage therapies. Phage therapy can be an effective treatment for antibiotic-resistant bacteria, which pose a significant threat to the CF community.
A number of phages have been identified, categorized, developed, and now introduced into clinical trials. Phage therapy is particularly compelling because it can be used to treat multidrug-resistant bacteria when all other antibiotics fail. Antibiotic resistance constitutes a pressing challenge for the CF community due to their frequent use of antibiotics to treat their chronic lung infections.
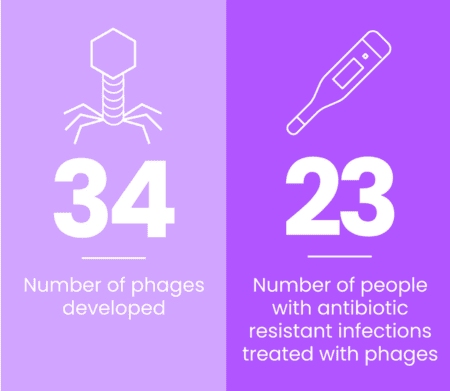
Thanks to EE’s grant funding, 34 phages were developed for the treatment of drug-resistant infections in people with CF by the end of 2023. In that time, the phages treated 23 individuals with a variety of antibiotic-resistant infections, including joint and pulmonary. Highlights of the work around phages include:
- In April 2023, EE Grant Recipient, David Pride, MD, PhD, and his team at the Center for Innovative Phage Applications and Therapeutics Lab (IPATH) identified and characterized more than 10 methicillin-resistant Staphylococcus aureus (MRSA) phages that can potentially be used to treat people with and without CF who have hard-to-treat MRSA infections. This work comes from a 2020 grant awarded by EE to Dr. Pride and his team to build a library of bacteriophages to treat people with CF who have MRSA infections.
- In July 2023, EE Grant Recipient Graham Hatfull, PhD, and his team at the University of Pittsburgh identified and characterized three phages that can potentially be used to treat people with nontuberculosis mycobacteria (NTM) infections The work was funded by a 2022 grant from EE to identify the treatment of NTM infections in people with CF.
- In August 2023, Yale University treated the first CF patient using a phage therapy that was developed with a 2019 grant from EE.
The successful development of phages is just one example of how EE grant recipients are transforming research projects into potential therapies for people with CF who need them most.
Innovative Research Approaches in CF Treatment
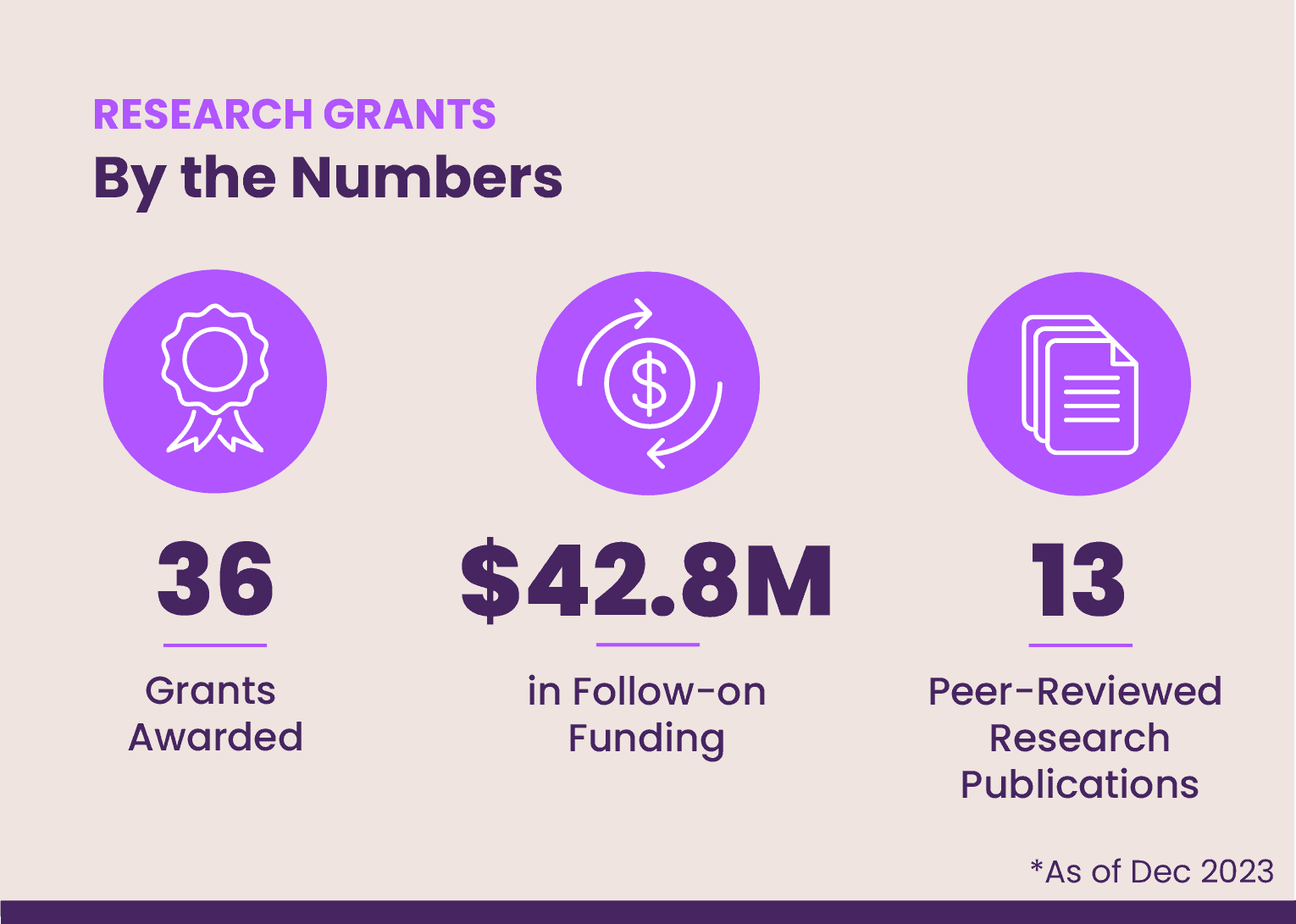
As of 2023, EE has awarded 36 grants to researchers from six countries and helped to secure more than $42.8 million dollars in follow-on funding across a variety of research categories. This includes three rounds of grants totaling nearly $2.5 million in 2022 and 2023 with research focused on the development of promising new gene-based therapies and drug-delivery technologies, among other priority areas.
In 2022 and 2023, EE also introduced new grant funding mechanisms outlined in the Scientific Roadmap to amplify the impact of its research program. These include co-funded, public/private partnerships, and multi-principle investigator collaborative grants. One example is a grant EE co-funded with the Cystic Fibrosis Trust, the UK’s leading charity supporting people with CF. The partnership underscored EE’s commitment to forming collaborative funding relationships with other organizations to maximize its impact in areas that align with EE’s research priorities. The project also exemplified an innovative public/private partnership between academia and a biotechnology company aimed at accelerating therapeutic development.
In calls for grant funding, EE received a record number of applications, demonstrating that the organization’s funding is filling a critical gap in the field, and there is significant—and growing—interest in working with EE as a funding partner. Submissions for grants in 2022 and 2023 came from across the globe and spanned all priority funding areas, underscoring the global interest and engagement in EE’s work.
2023 Grant Recipients
- Development of a partially humanized mouse airway for testing the efficiency of gene delivery methods (Andrew Berical, MD, Boston University Chobanian & Avedisian School of Medicine)
- Investigation of novel strategies for correction of a rare CFTR gene variant in CF (Luca Clarke, PhD, University of Lisboa, Portugal)
- Optimization of multifunctional ionizable lipids with the goal to provide a safe and efficient delivery platform for repeated local and systemic delivery of various nucleic acid therapeutics for treatment. (Zheng-Rong Lu, PhD and Mitchell Drumm, PhD, Case Western Reserve University)
- Development of polymeric vehicles to improve the delivery of nucleic acid-based therapeutics to target tissues and cells in the body, primarily the lung and the gastrointestinal tract. (Alexandra S. Piotrowski-Daspit, PhD, University of Michigan)
- Potential of a novel combinatorial treatment for people with CF made up of three approved drugs based on promising organoid and clinical data. (Jeffrey Beekman, PhD, University Medical Center Utrecht)




2022 Grant Recipients
- Ferret model used to test whether treatment using multiple drugs can reduce disease symptoms in the ferret and thus accelerate the identification of effective and safe therapeutic approaches for those with CF nonsense mutations. (Xinsen Sun, PhD, University of Iowa)
- Gene-editing therapies that may be able to repair the defective gene associated with CF. (Debadyuti Rana Ghosh, PhD and Hugh Smyth, PhD, University of Texas at Austin)
- Mitochondrial dysfunction in those with CF. (John G. Geisler, PhD, CSO and Robert Alonso, MBA, CEO and co-founder of Mitochon Pharmaceuticals, Inc.)
- Restoring CFTR protein function in nonsense mutations of CF through the discovery and validation of novel targets (Carlos M. Farinha, PhD, of the Biosystems and Integrative Sciences Institute at the Faculty of Sciences, University of Lisboa)
- Use of a novel gene editing technology, called prime editing, to correct mutations within the CFTR gene in people with CF. (Marianne S. Carlon, PhD, and Mattijs Bulcaen, KU Leuven in Belgium)
- Use of bacteriophages, which are viruses that infect bacteria, as a treatment for nontuberculosis mycobacterium (NTM) infections. (Graham F. Hatfull, PhD, University of Pittsburgh)
- Correcting the CFTR protein in people with at least one nonsense mutation of CF (Patrick L. Sinn, PhD, University of Iowa)
- Advance the development of an inhaled gene therapy platform for CF using biological, non-viral nanoparticles called extracellular vesicles (EVs) that are produced from stem cells. (Lorraine Martin, PhD, Queen’s University Belfast and OmniSpirant Therapeutics)
- Use of ACE-tRNAs to produce fully functional CFTR proteins in people with nonsense mutations of CF (Jeffrey Beekman, PhD, and Sacha Spelier, University Medical Center Utrecht, and John D. Lueck, PhD, University of Rochester Medical Center)






Conseil consultatif scientifique
Under the visionary leadership of Kevin Foskett, PhD, Chair of the Department of Physiology at the Perelman School of Medicine at the University of Pennsylvania, EE’s Scientific Advisory Board (SAB) is composed of leading researchers, clinicians, and biopharmaceutical industry experts. The SAB is pivotal in identifying and guiding strategic research priorities for our organization. In the past two years, EE has expanded the SAB to include four distinguished scientists closely aligned with and committed to the mission of accelerating research and drug development for the final 10% of people with CF. These individuals include:
- David M. Bedwell, PhD, the James C. and Elizabeth T. Lee Endowed Chair of Biochemistry and professor and chair of the Department of Biochemistry and Molecular Genetics in the Heersink School of Medicine at the University of Alabama at Birmingham (UAB).
- Patrick Harrison, PhD, senior lecturer in molecular physiology and head of the CF Gene Editing Lab in the Department of Physiology, School of Medicine, at University College Cork, Ireland.
- Maria P. Limberis, PhD, the Vice President of Research at Spirovant Sciences, Inc.
- Margarida D. Amaral, PhD, a Full Professor of Biochemistry/Molecular Biology at the Faculty of Sciences, University of Lisboa (Portugal) and Group Leader at the Biosystems & Integrative Sciences Institute (BioISl).








Accelerating the Pathway Forward: The 2023 Scientific Symposium
Among EE’s greatest strengths is its ability to convene key players in the CF field and beyond to accelerate drug development. In December 2023, we hosted a global scientific symposium at Quorum at the University City Science Center in Philadelphia, Pennsylvania, entitled “The Promise of Gene-based Therapies for Cystic Fibrosis.”
Chaired by leading CF researchers Patrick Harrison, PhD, Maria Limberis, PhD, and Batsheva Kerem, PhD, the event brought together more than 34 speakers and 86 attendees from 13 countries, including leading minds from within and outside the CF scientific community, industry leaders, and federal Food and Drug Administration (FDA) representatives. The program featured a curated line-up of keynote plenaries and round-table discussions aimed at addressing the challenges that face the development of gene-based therapies for CF. The symposium aimed to foster open dialogue, address hurdles, and collaboratively devise solutions and strategic, accelerated pathways forward for the CF community and the final 10% in particular.




