
גיוס ניסויים קליניים
זירוז גיוס לניסויים קליניים
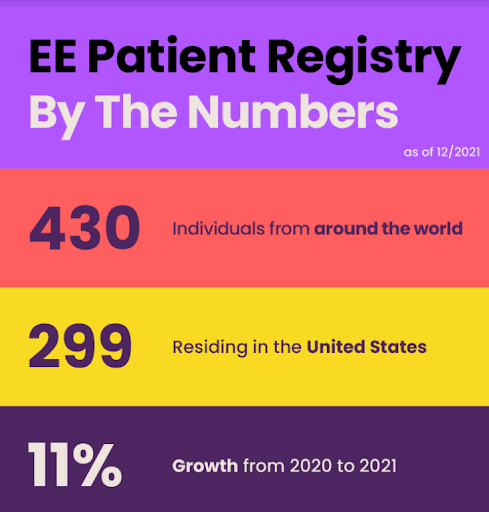
EE’s CF Nonsense Mutation Patient Registry ו תוכנית שידוכים לניסוי קליני continued to grow and yield important results in 2021. The registry expedites clinical trial recruitment by aggregating a pool of individuals with at least one copy of a nonsense mutation of CF who are interested in participating in clinical trials. EE’s matchmaking program then connects those individuals directly to relevant clinical research opportunities.
One of the major barriers to therapeutic development is clinical trial recruitment, which can be particularly costly, time-consuming, and inefficient. This holds particularly true for people with rare diseases and mutations, like many of those in the final 10% of the CF community, because the pool of clinical trial participants is so small and spread out.
EE’s efforts effectively streamline the recruitment process and provide multiple benefits to empower the community.
Additionally, the demographic data collected in the registry helps inform clinical trial site identification and selection based on where registrants live, allowing for a more strategic and equitable distribution of trials. EE can now advocate for companies to set up trials in parts of the country and world where there are eager, relevant patients that would not normally have access. This personalized, direct-to-consumer process is unique in the CF space and has the potential to yield significant results: greater representation and diversity among trial participants, greater access to trials for people who may not otherwise have had the option to participate, and faster trial enrollment (thereby leading to faster and more cost-effective drug development).
EE has developed a formalized intake process to accept and review requests and a comprehensive rubric to evaluate them. Due to significant interest in the clinical trial matchmaking program, EE launched a Clinical Research Review Committee, an advisory committee made up of clinical researchers, clinicians, and individuals with CF that reviews and assesses clinical trial partnership proposals from sponsors and investigators based on EE’s research goals and makes recommendations about which proposals should move forward and be prioritized.
Pilot Project with Translate Bio
This year, EE completed its first official pilot project with Translate Bio for the company’s MRT5005 mRNA trial using the registry data and clinical trial matchmaking program for clinical trial patient identification, outreach, and clinical site referral and coordination. EE identified and contacted 101 patients that appeared to fit eligibility criteria for the study based on their reported registry information. Based on this outreach, EE generated 12 referrals and one enrollment for this particular study, which was recruiting 40 participants total. To generate an enrollment for a global study targeting a small subset of an already rare disease is a major accomplishment and reflects the value, efficacy, and potential of the clinical trial matchmaking program. These are promising, tangible outcomes that have generated considerable excitement and anticipation about the future impact and scalability of the program.
Since the successful completion of this pilot, seven biotech companies have expressed interest in partnering with EE for clinical trial matching, suggesting that the program is filling a critical need in the field.
