
- Lar
- A história de Emily
- Sobre nós
- Pesquisar
- Eventos
- Imprensa e Mídia
- Tome uma atitude
- Blogue
- Contato
- Doar

Recrutamento para estudos clínicos
Acelerando o recrutamento para estudos clínicos
One of the major barriers in therapeutic development is clinical trial recruitment, which is costly, time-consuming, and inefficient. Emily’s Entourage has created and manages a patient registry of individuals with at least one copy of a CF nonsense mutation to address this barrier.
The patient registry provides two critical functions. The first is to allow individuals with nonsense mutations of CF to contribute their data and become informed of relevant research, clinical advances, and trials. The second is to enable faster, more targeted clinical trial recruitment and enrollment for companies and investigators, thereby expediting the development of lifesaving therapeutics. In addition, demonstrating a ready-made community eager to participate in clinical trials helps attract biopharmaceutical companies into the CF space and provides insights into where to launch clinical trial sites.
As of 2020, the EE patient registry consists of 387 individuals from around the world, with 268 of those individuals residing in the United States. EE grew the patient registry by 12% from 2019 to 2020.
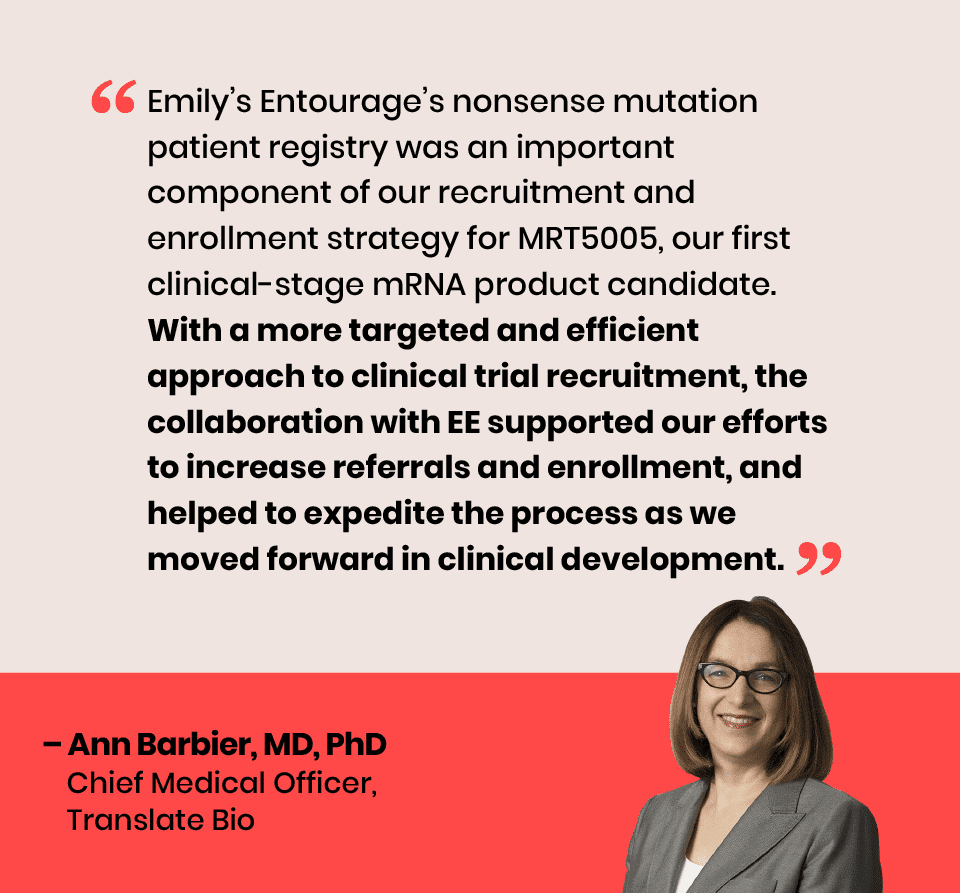
In October 2020, EE launched a first-of-its-kind collaboration with Translate Bio to pilot the patient registry as a tool for clinical trial patient identification and outreach. The partnership yielded increased screening and successful trial enrollments toward the company’s estimated enrollment goal of 40 participants.
As of December 2020, we are focused on growing the patient registry and our partnerships with sponsors and investigators. An international expansion to recruit more patients to the registry is underway and beginning with Israel given its high prevalence of individuals with nonsense mutations of CF.