
Förespråkande
Av, genom och för CF-communityn
Advocacy for and representation of the final 10% of the CF community are at the heart of EE’s mission. We tirelessly advocate for research and therapeutic development for those in the final 10% that do not benefit from current mutation-targeted therapies, using our platform, network, and reach to ensure that the diverse needs are heard and served. In recent years, we have amplified our efforts to bridge knowledge and data gaps in CF drug development by better understanding and incorporating the perspectives of stakeholders with leadership from people with CF and their families. This has been accomplished through various forms of community engagement, including EE’s “Final 10%” surveys in 2021 and 2022 that have provided critical, unprecedented data on the health and perspectives of people with CF that do not benefit from approved CFTR modulators.
Additionally, as a patient- and family-led research organization, the leadership team has a deep, vested interest in the outcomes EE seeks. That laser focus on bringing life saving treatments to people with CF drives the organization’s direction and pace and informs every decision that is made. The leadership also has an intimate understanding of the problems the organization is seeking to solve and the nuanced dynamics and needs of the CF community.
Final 10% Survey: Revealing the True Impact
In 2021, EE launched the “Final 10%” Survey, a comprehensive global survey on the health and perspectives of individuals in the final 10% of the CF community that do not benefit from approved CFTR modulators. The findings, published in the peer-reviewed medical journal, Pediatric Pulmonology, revealed that individuals who are ineligible, intolerant, or lack access to CFTR modulators are experiencing a high burden of disease on their health. EE made this journal article open access to enable everyone in the CF community to learn and benefit from the findings.
Building on the success of the “Final 10%” 2021 survey, EE conducted the survey again in 2022. This time, we engaged the CF community by opening a submission period for question suggestions, receiving input from a diverse range of stakeholders, including people with CF, CF family members, clinicians, researchers, companies, and regulators.
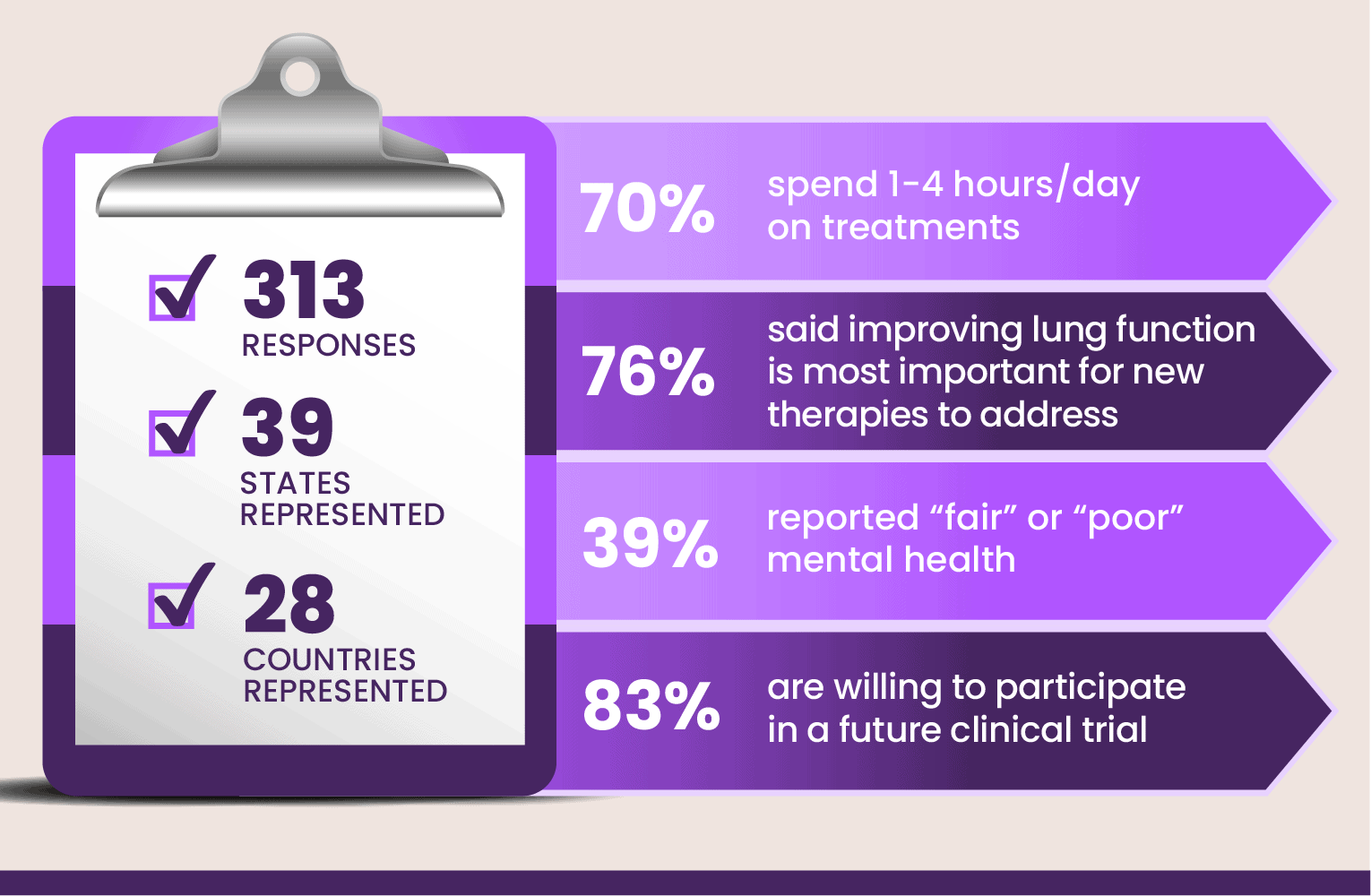
The 2022 survey generated 313 responses from 39 states and 28 countries, reaffirming the findings from 2021: people with CF not benefiting from modulators continue to experience substantial symptoms and treatment burden, adversely affecting their quality of life and their mental health. The knowledge that there are life-changing medications available to some, but not all, people with CF further adds to their distress.
Despite these challenges, the altruism of the CF community shines through. Many individuals not eligible for modulators are eager to participate in clinical trials, driven both by the hope of personal benefit and a desire to help others with CF.
Gene Therapy Town Hall
In August 2023, EE hosted a virtual town hall meeting focused on gene-based therapies for CF. This virtual event aimed to empower the CF community with knowledge about gene-based therapy trials and provide a venue for attendees to ask questions and receive answers from expert CF clinicians from some of the biggest CF clinics in the United States. Questions were submitted in advance (more than 400 questions/responses were submitted) and also live during the town hall. A total of 113 people from 5 different countries attended the live event, representing the concerns and hopes of the diverse CF community. Post event surveys showed that attendees felt significantly more prepared and reported being more likely to pursue gene therapy trials following the event.

National Conferences
EE had a presence at several key national conferences, hosting booths at Cystic Fibrosis Research Institute’s (CFRI’s) annual conference and the North American Cystic Fibrosis Conference (NACFC) in 2022 and 2023 to raise awareness about EE and recruit people with CF to sign up for its patient database. At NACFC, EE also held its annual reception to honor and celebrate the scientific community. Both years, the event brought together over 100 researchers, clinicians, industry representatives, and community members and included the presentation of the Trailblazer Award, which recognizes innovative changemakers in CF research, drug development, and care, to Jennifer Taylor-Cousar, MD, MSCS, ATSF in 2022 och Denis Hadjiliadis, MD, in 2023.


