
Rekrytering av kliniska prövningar
A New Era for Clinical Trial Recruitment
Clinical trial recruitment is a major barrier to therapeutic development for rare diseases, and EE is at the forefront of addressing this challenge. Various therapies are approaching clinical trials for those in the final 10% of the CF community. To reduce delays in clinical trial recruitment and achieve sufficient trial participation, innovative strategies for reaching and engaging this ultra-rare population are essential.
Pioneering New Recruitment Strategies to Speed Recruitment
Unlike other CF patient registries, EE’s global patient database is direct-to-consumer, allowing us to connect individuals directly with clinical trial opportunities rather than solely going through care centers or providers. This approach embodies our belief that people with CF should be empowered to take charge of their own health by having access to information that enables them to make decisions about clinical research and care. It also promotes more strategic and equitable distribution of trials and better representation among participants.
*It should be noted that previously, the database was only for people with one or two copies of a CF nonsense mutation. As of 2024, EE significantly expanded the database to anyone that is not benefitting from a CFTR modulator, due to genetic ineligibility, suboptimal response, or side effects.
Elevating The Database Platform: Improving the User Experience
In 2023, EE significantly upgraded the technology platform to support global growth of our patient database. After an extensive, year-long search, we selected and custom-built a platform designed to enhance both the front-end and back-end user experiences. The new platform allows EE to generate queries, pull reports, and analyze the data with greater efficiency and sophistication, enhancing its ability to understand and communicate the data and ultimately improving its ability to serve the CF community. Additionally, the platform supports the program’s global expansion with compliance with international data and privacy laws.
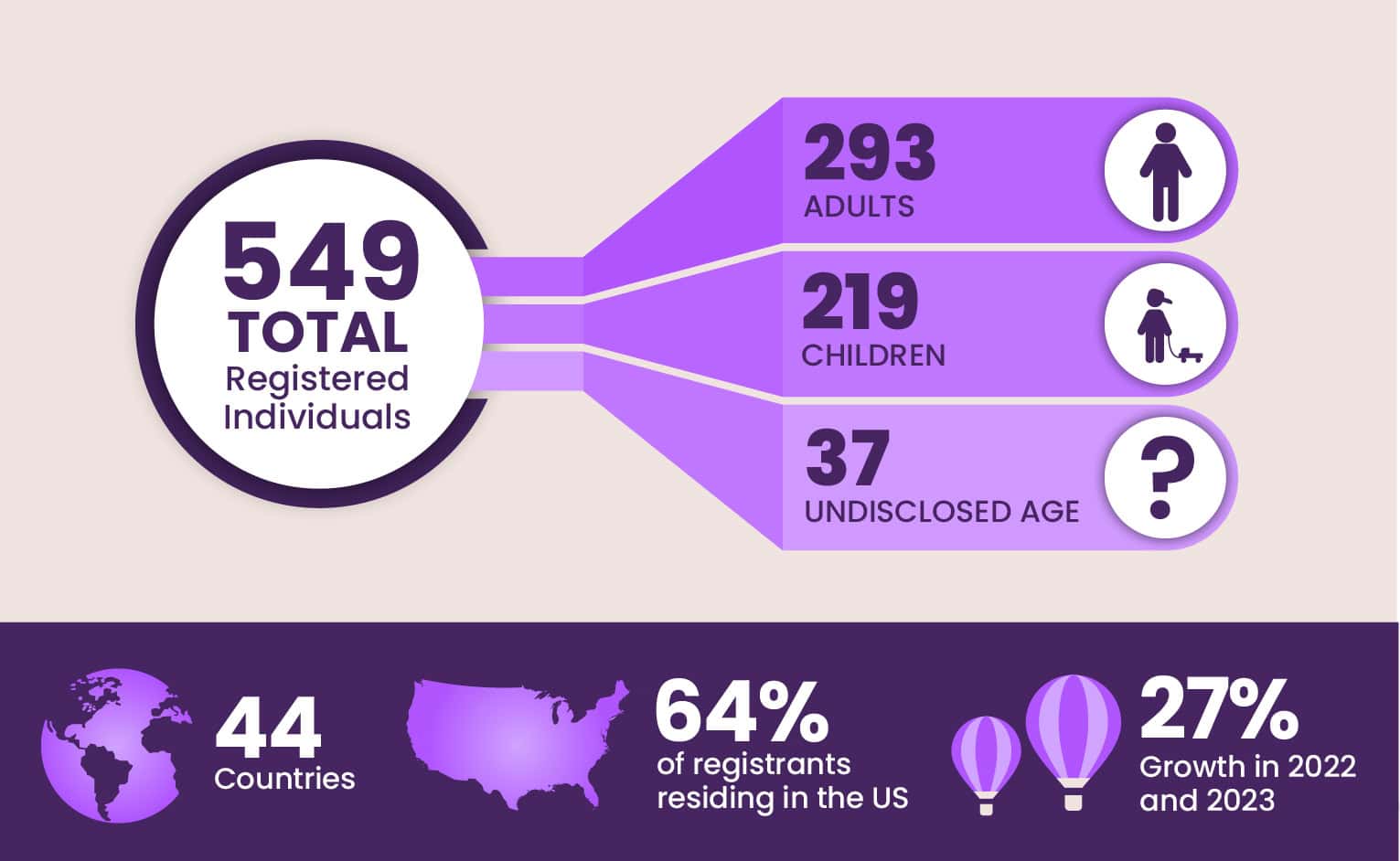
The efforts to expand the database are paying off. As of December 2023, the EE patient database had grown to include 549 individuals from 44 countries with approximately 64% of registrants residing in the United States. The database includes 293 adults, 219 children, and 37 participants with an undisclosed age. The database grew by 27% in 2022 and 2023.
Expansion of EE’s Clinical Trial Recruitment Program
EE continues to make significant inroads developing and expanding its clinical trial matchmaking program. Through EE’s clinical trial matchmaking program, EE shares relevant opportunities directly with individuals on the database. EE’s support makes clinical trial recruitment faster, more targeted, and more efficient, enabling more expedited drug development. Additionally, the matchmaking program equips individuals with CF with the information and tools to make their own choices about clinical research without relying on providers to make them aware of opportunities.
EE’s role in clinical trial matchmaking involves identifying people from its patient database who may meet the inclusion and exclusion criteria of a study based on self-reported data, outreach and communications about the study, and connecting any interested prospects with trial sites for further evaluation and possible enrollment.
EE-Supported Clinical Trials
In 2022 and 2023, EE supported the following sponsors and studies through its clinical trial matchmaking program:
- Aridis Pharmaceuticals’ AR-501 study, evaluating the safety and biological activity of an investigational drug called AR-501, an anti-infective molecule.
- Vertex Pharmaceuticals, Inc.’s BEACON CF study (SAD phase) of an investigational mRNA therapy for the final 10% of the CF community who do not benefit from CFTR modulators.
- 4DMT’s AEROW study, evaluating the safety, tolerability, and efficacy of 4D-710, an investigational gene therapy, in adults with CF lung disease who are ineligible or unable to tolerate CFTR modulator therapy.
EE’s clinical trial matchmaking services generated awareness and new referrals for clinical trials. There is demonstrated and growing interest from biotech and pharmaceutical companies in using EE’s database for recruitment support.
